Shop by Category
- Accessories
- Aspirator
- Cosmetics
- Digital Photography Systems
- Eyewear
- Footswitch/Foot Pedal
- Handpieces
- Infiltration Pump
- Laser Eyewear
- Microdermabrasion Devices
- Miscellaneous
- Portable Devices
- Skin Care Products and Make Up
- Skin Chillers
- Skin Sales Tools
- Smoke Evacuator
- Tips & Consumables
- Training & Business Tools
- User Manual/Operators Manual
- Les Encres
- Manufacturer
- Active Optical Systems
- Advalight
- Aerolase
- Aesthera
- Aesthetic Management Partners (AMP)
- Aesthetics Biomedical
- Aesthetika Lasers
- Alma Lasers
- Artas
- Asclepion
- Bella Products Inc.
- BTL Aesthetics
- Buffalo Filter
- Candela
- Canfield
- Cartessa
- Cellsound Aesthetics
- Cenmade
- Cervello
- Chromogenex
- CoolTouch
- Cutera, Inc.
- Cynosure, Inc.
- Deka
- Dermasweep
- DiamondTome
- Dornier
- Dusa
- Eclipse Aesthetics
- Edge Systems
- Eleme
- Ellman
- Emage Medical
- Emvera
- EndyMed
- Energist
- Equipmed
- Erchonia
- Fisioline
- Focus Medical
- Formatk
- Fotona
- Fraxel
- HK Surgical
- HOYA ConBio
- Human Med
- Hydrafacial
- Ilooda
- Inmode Aesthetic
- Iridex
- Jeisys
- Lasering
- LaserOptek
- Laserscope / Iridex
- Lazerlenz
- Light Bioscience
- Lipo Ltd
- Lumenis
- Lutronic, Inc.
- Mattioli
- Medical Laser Technolgies
- Medicamat
- MeDioStar NEXT
- Merz
- Miramar Labs
- NeoGraft
- Noir
- Noir Laser Eyewear
- Novoxel
- Nuvolase
- Nylo Aesthetics
- Obagi Medical
- Omnilux
- Other
- Palomar Medical
- Parisian Peel
- Photo Therapeutics
- Profect Medical Technologies
- Quanta Systems
- Quantel
- RA Medical
- Radiancy
- Reveal
- RevecoMed
- Rohrer Aesthetics
- Sandstone
- Sano Laser
- Sciton, Inc.
- Sharplight
- Sincoheren
- Solta Medical
- Storz Medical
- SupraMedical
- Sybaritic, Inc
- Syneron, Inc.
- Syntech Laser
- Thermage, Inc.
- Thermi Aesthetics
- Ulthera, Inc
- Ultra Aesthetica
- Venus Concepts
- Vibraderm
- Viora
- Vivace
- Viveve
- Wontech
- Zeltiq
- Zimmer
- Procedure
- Acne Scars
- Acne Treatment
- Acoustic Radial Shockwaves
- Actinic Keratosis
- Age Spots
- Body Contouring
- Body Contouring – Diode
- Body Contouring – Hands Free
- Body Contouring – Radio Frequency
- Body Contouring – Ultrasound & Radio Frequency Combined
- Cavitation
- Cellulite Smoothing
- Cherry Angiomas
- Collagen Regeneration
- Complexion Analysis
- Dental
- Dry Eye
- Dynamic Muscle Stimulation
- Epidermal Renewal
- Erectile Dysfunction
- Face Contouring
- Face Oil Reduction
- Facial
- Facial Muscle Stimulation Treatment
- Facial Redness
- Fat & Cellulite Reduction
- Fat Grafting
- Fractional Skin Resurfacing
- Freckles
- Hair Restoration
- Hyperhidrosis
- Hyperpigmentation
- Incontinence
- Keloid Scar Treatment
- Laser Hair Removal
- Laser Hair Removal – Diode
- Laser Lipolysis
- Liposuction
- Lymphatic Massage/Drainage
- Melanin Reduction
- Melasma Removal
- Microdermabrasion
- Microneedling
- Muscle Sculpting
- Non-Invasive Hair Growth Treatment
- Non-Surgical
- Oxygen Skin Therapy
- Pain Relief
- Photodynamic Therapy
- Photofacial Rejuvenation
- Pigment Reduction
- Pigmented Lesion
- Podiatry
- Pore Minimizer – Minimize Enlarged Pores
- Port Wine Stains
- Psoriasis
- Reverse Photo-aging Damage
- RF Microneedling
- Rosacea Treatment
- Scalp Health
- Scar Correction
- Seborrheic Keratosis
- Skin Analysis
- Skin Chilling
- Skin Rejuvenation – Ablative
- Skin Rejuvenation – Non-Ablative
- Skin Rejuvenation – Sublative
- Skin Resurfacing
- Skin Tags
- Skin Tightening
- Skin Tightening – Eye Area
- Spider Veins
- Sterilize
- Stretch Marks Removal
- Sun Spots
- Surgical
- Sweat Reducer
- Tattoo Removal
- Toenail Fungus
- Transepidermal Delivery
- Ulcer/Wound Healing
- Vaginal Rejuvenation
- Vascular Lesions
- Vascular Structure
- Vein Removal
- Warts
- Wrinkle Reduction
- User Manuals
- Wavelength
- 10,600 nm (CO2)
- 1060 nm (Diode)
- 1064 nm (Diode)
- 1064 nm (Nd Yag)
- 1064 nm & 755 nm
- 1064 nm, 755 nm & IPL
- 1100 – 1800 nm (Infrared)
- 1319 nm (Infrared Nd Yag)
- 1320 nm (Infrared Nd Yag)
- 1400 nm (Erbium Glass)
- 1440 nm Nd Yag
- 1450 nm (Diode)
- 1470 nm
- 1500 nm (Erbium Glass)
- 1540 nm Erbium Glass
- 1550 nm Erbium Glass
- 1565nm
- 1750nm (Infrared)
- 1927 nm (Thulium)
- 2790 nm (Erbium YSGG)
- 2940 nm (Erbium Yag)
- 308 nm (Xenon Chloride)
- 532 nm (KTP / Q-Switched)
- 585 nm (Pulsed Dye)
- 595 nm (Pulsed Dye)
- 635 nm (Diode)
- 650-660 nm (Diode)
- 670 nm
- 694 nm (Ruby)
- 700 nm
- 755 nm (Alexandrite)
- 800 nm (Diode)
- 805 nm (Diode)
- 808 nm
- 810 nm (Diode)
- 850nm (Infrared)
- 900 nm (Diode)
- Acoustic Wave Therapy AWT
- AFT (Advanced Fluorescence Technology)
- AMP – Active Magnetic Pulse
- CO2
- Direct Bio-Electrical Muscle Stimulation
- ElectoFusion
- EMS (Electrical Muscle Stimulation)
- HIFEM (High Intensity Focused
- IPL (Broad Spectrum)
- LED (Light Emitted Diodes)
- LIESWT – Low- Intensity Extracorporeal Shock Wave Therapy
- Multi-Wavelength
- Nitrogen Plasma
- Radio Frequency
- Radio Frequency – Bipolar
- Radio Frequency – Monopolar
- Radio Frequency – Unipolar
- Shock Wave Therapy
- TMA – Thermo-Mechanical Action
- Tripollar RF
- Ultrasound
- Ultrasound & Radio Frequency
Home / Products / Wavelength / Ultrasound / 2015 Syneron Candela Ultrashape Ultrasound – Seller Has Agreed to Represent (km/mil)x
2015 Syneron Candela Ultrashape Ultrasound – Seller Has Agreed to Represent (km/mil)x
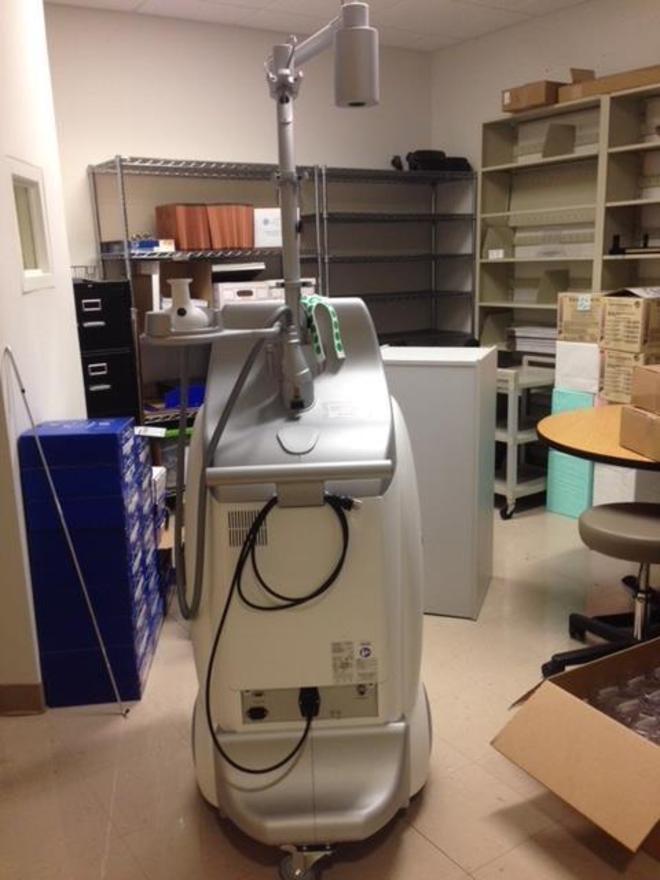
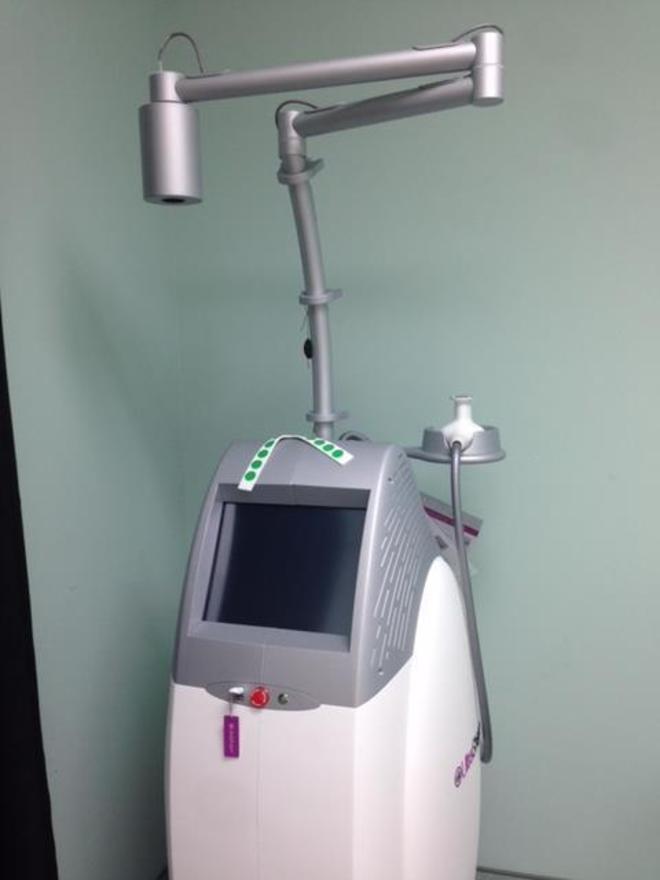
2015 Syneron Candela Ultrashape Ultrasound Body Contouring – Seller Has Agreed to Represent the Equipment for a Period of 2 Years for the Purchase of FTZ Cycles, Treatment Kits and Service; Excellent Cosmetic & Operating Condition, Single Owner, Full Maintenance Service and Certification Performed 09/27/2018, Records Available Upon Request; Includes: Large Applicator, U-Sculpt Transducer/Applicator/Handpiece, Brochures, Drainage Tool, Patient Straps, Red Bulls Eye Stickers, Operators Manual, and 90 Day Warranty. (km/mil3.5)
$4,975.00
Out of stock
| Product Specifications | |
|---|---|
| Certifications: | FDA clearance, CE (0344) mark, CSA, CB Scheme |
| Focused ultrasound technology: | Ultrasound operation frequency: 200 ± 30 KHz |
| Input voltage: | Set by technician during installation: 110-120 V or 200-240 V, 50/60 Hz |
| Dimensions: | 200 x 98 x 65 cm (H x L x W) |
| Weight: | System Console: Less than 130 kg |
| Ultrasound Transducer: | Less than 2.5 kg |
| Manufacturer Notes |
|---|
What can you treat with UltraShape?
What will your patients experience?
What are the main advantages for your practice?
Fast Results and Comfortable Treatment with a Clinically Proven, Non-Invasive Body Contouring Technology. The UltraShape® System is the first and only FDA cleared, non-invasive body shaping device that uses pulsed, focused ultrasound to mechanically (non-thermally) and selectively destroy fat cells at a designated focal point in the subcutaneous fat tissue without harming the skin, blood vessels, nerves or connective tissue, demonstrating measurable results in as little as 2 weeks. This unique technology answers the growing patient demand for a non-invasive body shaping solution that offers no downtime and without the complications associated with surgery. UltraShape – Focused on Results
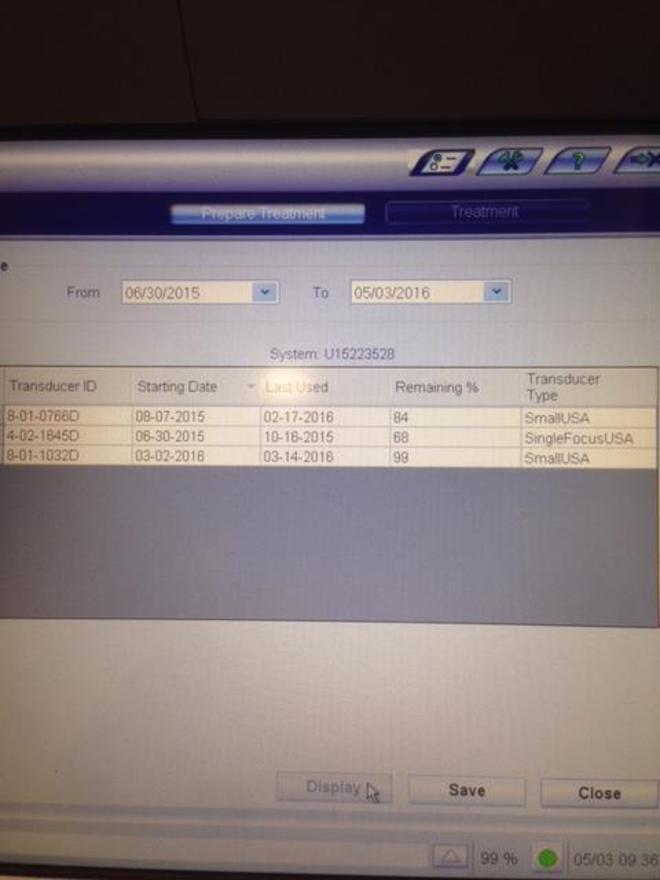
Consumables Required: FTZ Cycles & Treatment Kit; Transducers/Applicators are not a consumable; they are continuously reusable like normal laser handpieces. Syneron Candela Charges a Recertification Fee of $30,000 for the Purchase of Consumables. |
| Date Sold |
|---|
* Shipping is included in the Continental USA only. Any shipment outside the lower 48 United States will be shipped at an extra cost. If you live outside this area, please contact us for a free shipping estimate.
Related Products
Related products
-
2005 LuxRs Handpiece for Palomar Starlux 300 – Hair Removal – Free Shipping (km/oka)s
$6,500.00Original price was: $6,500.00.$1,150.00Current price is: $1,150.00. Read more