Shop by Category
- Accessories
- Aspirator
- Cosmetics
- Digital Photography Systems
- Eyewear
- Footswitch/Foot Pedal
- Handpieces
- Laser Eyewear
- Microdermabrasion Devices
- Miscellaneous
- Portable Devices
- Skin Care Products and Make Up
- Skin Chillers
- Skin Sales Tools
- Smoke Evacuator
- Tips & Consumables
- Training & Business Tools
- User Manual/Operators Manual
- Les Encres
- Manufacturer
- Active Optical Systems
- Aerolase
- Aesthera
- Aesthetic Management Partners (AMP)
- Aesthetics Biomedical
- Aesthetika Lasers
- Alma Lasers
- Artas
- Asclepion
- Bella Products Inc.
- BTL Aesthetics
- Buffalo Filter
- Candela
- Canfield
- Cartessa
- Cellsound Aesthetics
- Cenmade
- Cervello
- Chromogenex
- CoolTouch
- Cutera, Inc.
- Cynosure, Inc.
- Deka
- Dermasweep
- DiamondTome
- Dornier
- Dusa
- Eclipse Aesthetics
- Edge Systems
- Eleme
- Ellman
- Emage Medical
- Emvera
- EndyMed
- Energist
- Equipmed
- Erchonia
- Fisioline
- Focus Medical
- Formatk
- Fotona
- Fraxel
- HOYA ConBio
- Human Med
- Hydrafacial
- Ilooda
- Inmode Aesthetic
- Iridex
- Jeisys
- Lasering
- LaserOptek
- Laserscope / Iridex
- Lazerlenz
- Light Bioscience
- Lipo Ltd
- Lumenis
- Lutronic, Inc.
- Mattioli
- Medical Laser Technolgies
- Medicamat
- MeDioStar NEXT
- Merz
- Miramar Labs
- NeoGraft
- Noir
- Noir Laser Eyewear
- Novoxel
- Nuvolase
- Nylo Aesthetics
- Obagi Medical
- Omnilux
- Other
- Palomar Medical
- Parisian Peel
- Photo Therapeutics
- Profect Medical Technologies
- Quanta Systems
- Quantel
- RA Medical
- Radiancy
- Reveal
- RevecoMed
- Rohrer Aesthetics
- Sandstone
- Sano Laser
- Sciton, Inc.
- Sharplight
- Solta Medical
- Storz Medical
- SupraMedical
- Sybaritic, Inc
- Syneron, Inc.
- Syntech Laser
- Thermage, Inc.
- Thermi Aesthetics
- Ulthera, Inc
- Ultra Aesthetica
- Venus Concepts
- Vibraderm
- Viora
- Vivace
- Viveve
- Wontech
- Zeltiq
- Zimmer
- Procedure
- Acne Scars
- Acne Treatment
- Actinic Keratosis
- Body Contouring
- Body Contouring – Diode
- Body Contouring – Hands Free
- Body Contouring – Radio Frequency
- Body Contouring – Ultrasound & Radio Frequency Combined
- Cellulite Smoothing
- Collagen Regeneration
- Complexion Analysis
- Dental
- Dry Eye
- Dynamic Muscle Stimulation
- Epidermal Renewal
- Erectile Dysfunction
- Face Contouring
- Face Oil Reduction
- Facial
- Facial Muscle Stimulation Treatment
- Fat & Cellulite Reduction
- Fat Grafting
- Fractional Skin Resurfacing
- Hair Restoration
- Hyperhidrosis
- Hyperpigmentation
- Incontinence
- Keloid Scar Treatment
- Laser Hair Removal
- Laser Hair Removal – Diode
- Laser Lipolysis
- Liposuction
- Lymphatic Massage/Drainage
- Melanin Reduction
- Melasma Removal
- Microdermabrasion
- Microneedling
- Muscle Sculpting
- Non-Invasive Hair Growth Treatment
- Non-Surgical
- Oxygen Skin Therapy
- Pain Relief
- Photodynamic Therapy
- Photofacial Rejuvenation
- Pigment Reduction
- Pigmented Lesion
- Podiatry
- Pore Minimizer – Minimize Enlarged Pores
- Psoriasis
- Reverse Photo-aging Damage
- RF Microneedling
- Rosacea Treatment
- Scalp Health
- Scar Correction
- Seborrheic Keratosis
- Skin Analysis
- Skin Chilling
- Skin Rejuvenation – Ablative
- Skin Rejuvenation – Non-Ablative
- Skin Rejuvenation – Sublative
- Skin Resurfacing
- Skin Tightening
- Skin Tightening – Eye Area
- Sterilize
- Stretch Marks Removal
- Surgical
- Sweat Reducer
- Tattoo Removal
- Toenail Fungus
- Transepidermal Delivery
- Ulcer/Wound Healing
- Vaginal Rejuvenation
- Vascular Lesions
- Vascular Structure
- Vein Removal
- Warts
- Wrinkle Reduction
- User Manuals
- Wavelength
- 10,600 nm (CO2)
- 1060 nm (Diode)
- 1064 nm (Diode)
- 1064 nm (Nd Yag)
- 1064 nm & 755 nm
- 1064 nm, 755 nm & IPL
- 1100 – 1800 nm (Infrared)
- 1319 nm (Infrared Nd Yag)
- 1320 nm (Infrared Nd Yag)
- 1400 nm (Erbium Glass)
- 1440 nm Nd Yag
- 1450 nm (Diode)
- 1470 nm
- 1500 nm (Erbium Glass)
- 1540 nm Erbium Glass
- 1550 nm Erbium Glass
- 1565nm
- 1750nm (Infrared)
- 1927 nm (Thulium)
- 2790 nm (Erbium YSGG)
- 2940 nm (Erbium Yag)
- 308 nm (Xenon Chloride)
- 532 nm (KTP / Q-Switched)
- 585 nm (Pulsed Dye)
- 595 nm (Pulsed Dye)
- 635 nm (Diode)
- 650-660 nm (Diode)
- 670 nm
- 694 nm (Ruby)
- 700 nm
- 755 nm (Alexandrite)
- 800 nm (Diode)
- 805 nm (Diode)
- 810 nm (Diode)
- 850nm (Infrared)
- 900 nm (Diode)
- Acoustic Wave Therapy AWT
- AFT (Advanced Fluorescence Technology)
- AMP – Active Magnetic Pulse
- CO2
- Direct Bio-Electrical Muscle Stimulation
- ElectoFusion
- EMS (Electrical Muscle Stimulation)
- HIFEM (High Intensity Focused
- IPL (Broad Spectrum)
- LED (Light Emitted Diodes)
- LIESWT – Low- Intensity Extracorporeal Shock Wave Therapy
- Multi-Wavelength
- Nitrogen Plasma
- Radio Frequency
- Radio Frequency – Bipolar
- Radio Frequency – Monopolar
- Radio Frequency – Unipolar
- Shock Wave Therapy
- TMA – Thermo-Mechanical Action
- Tripollar RF
- Ultrasound
- Ultrasound & Radio Frequency
Home / Products / Procedure / Vaginal Rejuvenation / 2018 Viveve RF Vaginal Rejuvenation
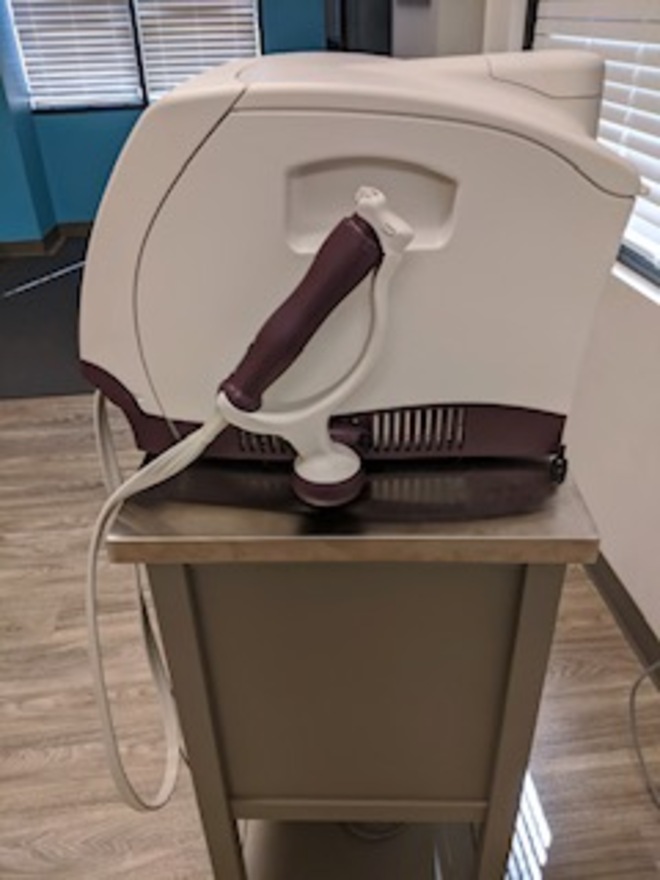
2018 Viveve RF Vaginal Rejuvenation
2018 Viveve RF Vaginal Rejuvenation; Manufactured 03/20/2018; Perfect Cosmetic & Operating Condition, Single Owner; Reason for Selling: Downsizing; Includes: Handpiece, Footswitch & 90 Day Warranty. (Viveve Does Not Charge A Recertification Fee for Service or the Purchase of Consumables). (jp/vin)
$85,000.00 Original price was: $85,000.00.$17,975.00Current price is: $17,975.00.
Out of stock
Laser Type:Radio Frequency, Monopolar Radio Frequency (CMRF)
Energy:90 J/cm2
Cooling:Cryogen
The Viveve System provides sustained tissue tightening and strengthening by substantially heating the tissue at depth. The Viveve System’s patented, cryogen-cooled monopolar radiofrequency (CMRF) is unique in its ability to deliver volumetric heat (90 J/cm2) in order to stimulate robust neocollagenesis. This can be accomplished in just ONE session.
The Viveve System is indicated for use in general surgery for electrocoagulation and hemostasis in the USA.
Quick Facts
Single Session
Cryogen-Cooled Monopolar Radiofrequency
One 30-45 minute session
Safe and comfortable
Clinically-proven
Respectable and discreet
Women worldwide are increasingly demanding nonsurgical treatments. However, there are a limited number of safe and effective technologies specifically designed for women’s intimate health. Viveve provides clinically proven, innovative, nonsurgical treatments to improve women’s intimate health.
The Viveve System delivers a single-session treatment to generate collagen and restore tissue. The dual mode VIVEVE TREATMENT COOLS AND PROTECTS THE SURFACE WHILE HEATING THE DEEPER TISSUE. The Viveve Treatment is both safe and effective.
The Viveve Treatment is delivered via the patented, cryogen-cooled monopolar radiofrequency (CMRF) device that rebuilds natural collagen. CMRF technology allows for depth of penetration while maintaining patient comfort and safety.
Viveve has completed enrolling a clinical trial for sexual dysfunction under an IDE.
Viveve also has a Stress Urinary Incontinence IDE under FDA review.
Viveve received approval of an Investigational Device Exemption (IDE) application from the U.S. Food and Drug Administration (FDA) in March of 2018 to proceed with VIVEVE II, a multicenter, randomized, double-blind, sham-controlled study to assess improvement of sexual function in women following childbirth.
Related Products
Related products
-
2010 BTL Aesthetics Exilis Ultra, Ultrasound & Radio Frequency for Skin Tightening & Skin Rejuvenation, with Face & Body Handpieces (jp/dan)
$95,000.00Original price was: $95,000.00.$22,975.00Current price is: $22,975.00. Read more