Shop by Category
- Accessories
- Aspirator
- Cosmetics
- Digital Photography Systems
- Eyewear
- Footswitch/Foot Pedal
- Handpieces
- Infiltration Pump
- Laser Eyewear
- Microdermabrasion Devices
- Miscellaneous
- Portable Devices
- Skin Care Products and Make Up
- Skin Chillers
- Skin Sales Tools
- Smoke Evacuator
- Tips & Consumables
- Training & Business Tools
- User Manual/Operators Manual
- Les Encres
- Manufacturer
- Active Optical Systems
- Advalight
- Aerolase
- Aesthera
- Aesthetic Management Partners (AMP)
- Aesthetics Biomedical
- Aesthetika Lasers
- Alma Lasers
- Artas
- Asclepion
- Bella Products Inc.
- BTL Aesthetics
- Buffalo Filter
- Candela
- Canfield
- Cartessa
- Cellsound Aesthetics
- Cenmade
- Cervello
- Chromogenex
- CoolTouch
- Cutera, Inc.
- Cynosure, Inc.
- Deka
- Dermasweep
- DiamondTome
- Dornier
- Dusa
- Eclipse Aesthetics
- Edge Systems
- Eleme
- Ellman
- Emage Medical
- Emvera
- EndyMed
- Energist
- Equipmed
- Erchonia
- Fisioline
- Focus Medical
- Formatk
- Fotona
- Fraxel
- HK Surgical
- HOYA ConBio
- Human Med
- Hydrafacial
- Ilooda
- Inmode Aesthetic
- Iridex
- Jeisys
- Lasering
- LaserOptek
- Laserscope / Iridex
- Lazerlenz
- Light Bioscience
- Lipo Ltd
- Lumenis
- Lutronic, Inc.
- Mattioli
- Medical Laser Technolgies
- Medicamat
- MeDioStar NEXT
- Merz
- Miramar Labs
- NeoGraft
- Noir
- Noir Laser Eyewear
- Novoxel
- Nuvolase
- Nylo Aesthetics
- Obagi Medical
- Omnilux
- Other
- Palomar Medical
- Parisian Peel
- Photo Therapeutics
- Profect Medical Technologies
- Quanta Systems
- Quantel
- RA Medical
- Radiancy
- Reveal
- RevecoMed
- Rohrer Aesthetics
- Sandstone
- Sano Laser
- Sciton, Inc.
- Sharplight
- Sincoheren
- Solta Medical
- Storz Medical
- SupraMedical
- Sybaritic, Inc
- Syneron, Inc.
- Syntech Laser
- Thermage, Inc.
- Thermi Aesthetics
- Ulthera, Inc
- Ultra Aesthetica
- Venus Concepts
- Vibraderm
- Viora
- Vivace
- Viveve
- Wontech
- Zeltiq
- Zimmer
- Procedure
- Acne Scars
- Acne Treatment
- Acoustic Radial Shockwaves
- Actinic Keratosis
- Age Spots
- Body Contouring
- Body Contouring – Diode
- Body Contouring – Hands Free
- Body Contouring – Radio Frequency
- Body Contouring – Ultrasound & Radio Frequency Combined
- Cavitation
- Cellulite Smoothing
- Cherry Angiomas
- Collagen Regeneration
- Complexion Analysis
- Dental
- Dry Eye
- Dynamic Muscle Stimulation
- Epidermal Renewal
- Erectile Dysfunction
- Face Contouring
- Face Oil Reduction
- Facial
- Facial Muscle Stimulation Treatment
- Facial Redness
- Fat & Cellulite Reduction
- Fat Grafting
- Fractional Skin Resurfacing
- Freckles
- Hair Restoration
- Hyperhidrosis
- Hyperpigmentation
- Incontinence
- Keloid Scar Treatment
- Laser Hair Removal
- Laser Hair Removal – Diode
- Laser Lipolysis
- Liposuction
- Lymphatic Massage/Drainage
- Melanin Reduction
- Melasma Removal
- Microdermabrasion
- Microneedling
- Muscle Sculpting
- Non-Invasive Hair Growth Treatment
- Non-Surgical
- Oxygen Skin Therapy
- Pain Relief
- Photodynamic Therapy
- Photofacial Rejuvenation
- Pigment Reduction
- Pigmented Lesion
- Podiatry
- Pore Minimizer – Minimize Enlarged Pores
- Port Wine Stains
- Psoriasis
- Reverse Photo-aging Damage
- RF Microneedling
- Rosacea Treatment
- Scalp Health
- Scar Correction
- Seborrheic Keratosis
- Skin Analysis
- Skin Chilling
- Skin Rejuvenation – Ablative
- Skin Rejuvenation – Non-Ablative
- Skin Rejuvenation – Sublative
- Skin Resurfacing
- Skin Tags
- Skin Tightening
- Skin Tightening – Eye Area
- Spider Veins
- Sterilize
- Stretch Marks Removal
- Sun Spots
- Surgical
- Sweat Reducer
- Tattoo Removal
- Toenail Fungus
- Transepidermal Delivery
- Ulcer/Wound Healing
- Vaginal Rejuvenation
- Vascular Lesions
- Vascular Structure
- Vein Removal
- Warts
- Wrinkle Reduction
- User Manuals
- Wavelength
- 10,600 nm (CO2)
- 1060 nm (Diode)
- 1064 nm (Diode)
- 1064 nm (Nd Yag)
- 1064 nm & 755 nm
- 1064 nm, 755 nm & IPL
- 1100 – 1800 nm (Infrared)
- 1319 nm (Infrared Nd Yag)
- 1320 nm (Infrared Nd Yag)
- 1400 nm (Erbium Glass)
- 1440 nm Nd Yag
- 1450 nm (Diode)
- 1470 nm
- 1500 nm (Erbium Glass)
- 1540 nm Erbium Glass
- 1550 nm Erbium Glass
- 1565nm
- 1750nm (Infrared)
- 1927 nm (Thulium)
- 2790 nm (Erbium YSGG)
- 2940 nm (Erbium Yag)
- 308 nm (Xenon Chloride)
- 532 nm (KTP / Q-Switched)
- 585 nm (Pulsed Dye)
- 595 nm (Pulsed Dye)
- 635 nm (Diode)
- 650-660 nm (Diode)
- 670 nm
- 694 nm (Ruby)
- 700 nm
- 755 nm (Alexandrite)
- 800 nm (Diode)
- 805 nm (Diode)
- 808 nm
- 810 nm (Diode)
- 850nm (Infrared)
- 900 nm (Diode)
- Acoustic Wave Therapy AWT
- AFT (Advanced Fluorescence Technology)
- AMP – Active Magnetic Pulse
- CO2
- Direct Bio-Electrical Muscle Stimulation
- ElectoFusion
- EMS (Electrical Muscle Stimulation)
- HIFEM (High Intensity Focused
- IPL (Broad Spectrum)
- LED (Light Emitted Diodes)
- LIESWT – Low- Intensity Extracorporeal Shock Wave Therapy
- Multi-Wavelength
- Nitrogen Plasma
- Radio Frequency
- Radio Frequency – Bipolar
- Radio Frequency – Monopolar
- Radio Frequency – Unipolar
- Shock Wave Therapy
- TMA – Thermo-Mechanical Action
- Tripollar RF
- Ultrasound
- Ultrasound & Radio Frequency
Home / Products / Wavelength / Ultrasound / 2022 Sofwave Medical Sofwave Non-Invasive Ultrasound Skin Tightening, Acne Scars & Wrinkle Reduction – Consumable Support, Free Shipping & Warranty Included (jp/men)
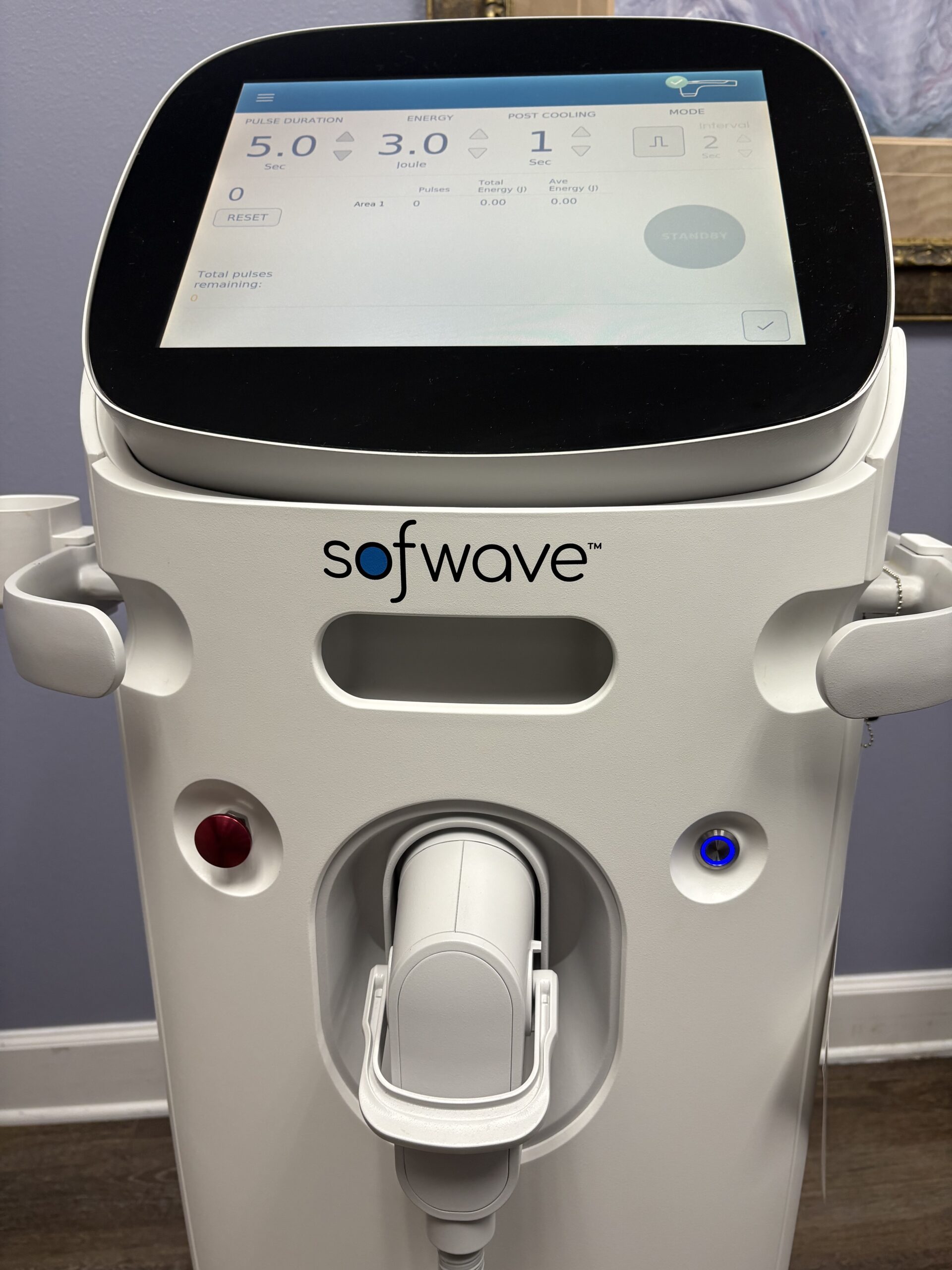
2022 Sofwave Medical Sofwave Non-Invasive Ultrasound Skin Tightening, Acne Scars & Wrinkle Reduction – Consumable Support, Free Shipping & Warranty Included (jp/men)
2022 Sofwave Medical Sofwave Non-Invasive Ultrasound Skin Tightening & Wrinkle Reduction;
Manufactured on 01/2022; Perfect Operating & Excellent Cosmetic Condition, Single Owner; Reason for Selling: No Longer Using;
Pulse Count: 16,899;
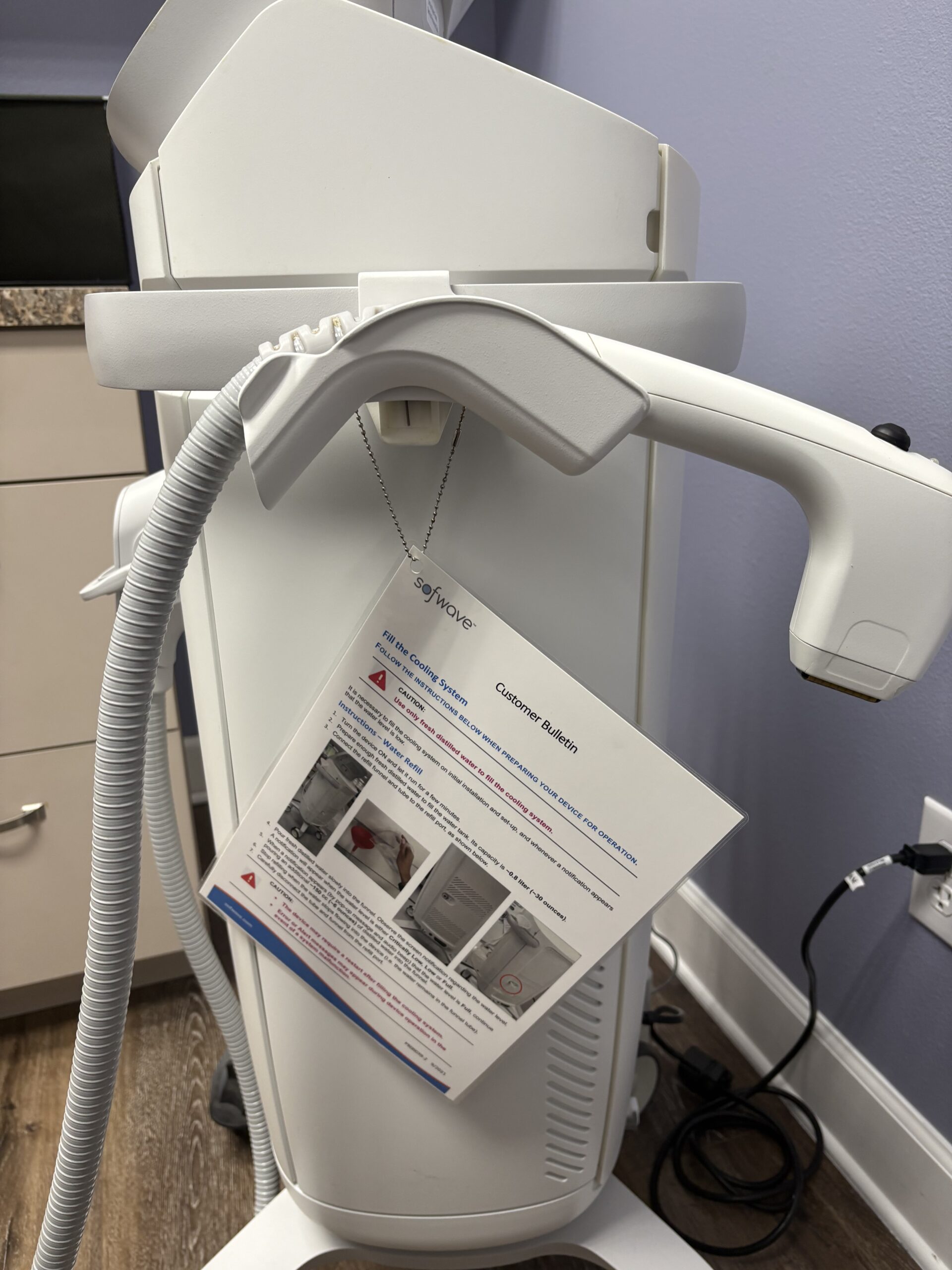
Includes: Handpiece, Footswitch, Interlock, Water Refill Kit, Operator Manual, Retractable Banner, (2) Before and After Flip Books, Brochures and Table Signs, Free Shipping and 90 Day Warranty;(jp/mer30)
Shipping to Canada is an Extra $250;
This Device can be Shipped Anywhere Around the World at an Extra Cost;
This Equipment is Fully Functional, With No Error Messages, Ready for Use, and Ready to Ship.
Warranty Includes Cost of Parts, Labor, & Shipping or Travel.
Financing Available. Training is Available at an Additional Cost.
See a Video of this Device Below:
$99,000.00 Original price was: $99,000.00.$38,975.00Current price is: $38,975.00.
Sofwave CONSUMABLES AND RECERTIFICATION
Consumables Required: Pulse Credits. Can be purchased through Sofwave Medical.
Does Sofwave MEDICAL Require a Recertification Fee for the Purchase of Consumables: Yes
Sofwave PRODUCT SPECIFICATIONS
Dimension approx: 122 × 40 × 40 cm (48″ × 15.75″ × 15.75″) in one brochure.
Weight: approx ~52 kg (114.6 lbs) in that same spec sheet.
Frequency: ~10-12 MHz ultrasound.
Electrical: 100-240 VAC, 50/60 Hz, 8 A max (as given in one spec sheet).
Sofwave MANUFACTURER NOTES
Cooling mechanism: The integrated “Sofcool™” active cooling system to protect the epidermis and monitor skin temperature.
Uses “Synchronous Ultrasound Parallel Beam (SUPERB™)” ultrasound technology.
Ultrasound beams are parallel, low-divergence, and typically there are 7 beams in the “Lift” applicator.
Target depth: mid-dermis around 1.5 mm beneath the skin surface.
The thermal effect is produced at roughly 60-70 °C (in mid-dermis) to stimulate collagen/elastin production.
Applicators / Handpieces
According to the FDA summary: there are multiple handpiece/applicator types: “Lift”, “Precise”, and in some filings “Smooth”.
Treatment area widths:
Precise applicator: ~15 mm width.
Lift applicator: ~35 mm width.
Smooth applicator (if included): ~70 mm width.
Energy per PZT (piezo-electric transducer) channel:
For Lift and Precise: ~3-5 J per channel.
For Smooth: ~2.6-7 J per channel.
Treatment Parameters / Use
Typical full face + neck treatment time: ~30–45 minutes.
Safe for all skin types (Fitzpatrick I-VI) because ultrasound is less affected by pigmentation than light/laser treatments.
Indications (approved uses)
Non-invasive dermatological aesthetic treatment to improve facial lines and wrinkles; lift eyebrow; lift lax submental (under the chin) and neck tissue.
Short-term improvement in the appearance of cellulite.
Treatment of skin laxity on upper arms (in newer clearance).
* Shipping is included in the Continental USA only. Any shipment outside the lower 48 United States will be shipped at an extra cost. If you live outside this area, please contact us for a free shipping estimate.