Shop by Category
- Accessories
- Aspirator
- Cosmetics
- Digital Photography Systems
- Eyewear
- Footswitch/Foot Pedal
- Handpieces
- Infiltration Pump
- Laser Eyewear
- Microdermabrasion Devices
- Miscellaneous
- Portable Devices
- Skin Care Products and Make Up
- Skin Chillers
- Skin Sales Tools
- Smoke Evacuator
- Tips & Consumables
- Training & Business Tools
- User Manual/Operators Manual
- Les Encres
- Manufacturer
- Active Optical Systems
- Advalight
- Aerolase
- Aesthera
- Aesthetic Management Partners (AMP)
- Aesthetics Biomedical
- Aesthetika Lasers
- Alma Lasers
- Artas
- Asclepion
- Bella Products Inc.
- BTL Aesthetics
- Buffalo Filter
- Candela
- Canfield
- Cartessa
- Cellsound Aesthetics
- Cenmade
- Cervello
- Chromogenex
- CoolTouch
- Cutera, Inc.
- Cynosure, Inc.
- Deka
- Dermasweep
- DiamondTome
- Dornier
- Dusa
- Eclipse Aesthetics
- Edge Systems
- Eleme
- Ellman
- Emage Medical
- Emvera
- EndyMed
- Energist
- Equipmed
- Erchonia
- Fisioline
- Focus Medical
- Formatk
- Fotona
- Fraxel
- HK Surgical
- HOYA ConBio
- Human Med
- Hydrafacial
- Ilooda
- Inmode Aesthetic
- Iridex
- Jeisys
- Lasering
- LaserOptek
- Laserscope / Iridex
- Lazerlenz
- Light Bioscience
- Lipo Ltd
- Lumenis
- Lutronic, Inc.
- Mattioli
- Medical Laser Technolgies
- Medicamat
- MeDioStar NEXT
- Merz
- Miramar Labs
- NeoGraft
- Noir
- Noir Laser Eyewear
- Novoxel
- Nuvolase
- Nylo Aesthetics
- Obagi Medical
- Omnilux
- Other
- Palomar Medical
- Parisian Peel
- Photo Therapeutics
- Profect Medical Technologies
- Quanta Systems
- Quantel
- RA Medical
- Radiancy
- Reveal
- RevecoMed
- Rohrer Aesthetics
- Sandstone
- Sano Laser
- Sciton, Inc.
- Sharplight
- Sincoheren
- Solta Medical
- Storz Medical
- SupraMedical
- Sybaritic, Inc
- Syneron, Inc.
- Syntech Laser
- Thermage, Inc.
- Thermi Aesthetics
- Ulthera, Inc
- Ultra Aesthetica
- Venus Concepts
- Vibraderm
- Viora
- Vivace
- Viveve
- Wontech
- Zeltiq
- Zimmer
- Procedure
- Acne Scars
- Acne Treatment
- Acoustic Radial Shockwaves
- Actinic Keratosis
- Age Spots
- Body Contouring
- Body Contouring – Diode
- Body Contouring – Hands Free
- Body Contouring – Radio Frequency
- Body Contouring – Ultrasound & Radio Frequency Combined
- Cavitation
- Cellulite Smoothing
- Cherry Angiomas
- Collagen Regeneration
- Complexion Analysis
- Dental
- Dry Eye
- Dynamic Muscle Stimulation
- Epidermal Renewal
- Erectile Dysfunction
- Face Contouring
- Face Oil Reduction
- Facial
- Facial Muscle Stimulation Treatment
- Facial Redness
- Fat & Cellulite Reduction
- Fat Grafting
- Fractional Skin Resurfacing
- Freckles
- Hair Restoration
- Hyperhidrosis
- Hyperpigmentation
- Incontinence
- Keloid Scar Treatment
- Laser Hair Removal
- Laser Hair Removal – Diode
- Laser Lipolysis
- Liposuction
- Lymphatic Massage/Drainage
- Melanin Reduction
- Melasma Removal
- Microdermabrasion
- Microneedling
- Muscle Sculpting
- Non-Invasive Hair Growth Treatment
- Non-Surgical
- Oxygen Skin Therapy
- Pain Relief
- Photodynamic Therapy
- Photofacial Rejuvenation
- Pigment Reduction
- Pigmented Lesion
- Podiatry
- Pore Minimizer – Minimize Enlarged Pores
- Port Wine Stains
- Psoriasis
- Reverse Photo-aging Damage
- RF Microneedling
- Rosacea Treatment
- Scalp Health
- Scar Correction
- Seborrheic Keratosis
- Skin Analysis
- Skin Chilling
- Skin Rejuvenation – Ablative
- Skin Rejuvenation – Non-Ablative
- Skin Rejuvenation – Sublative
- Skin Resurfacing
- Skin Tags
- Skin Tightening
- Skin Tightening – Eye Area
- Spider Veins
- Sterilize
- Stretch Marks Removal
- Sun Spots
- Surgical
- Sweat Reducer
- Tattoo Removal
- Toenail Fungus
- Transepidermal Delivery
- Ulcer/Wound Healing
- Vaginal Rejuvenation
- Vascular Lesions
- Vascular Structure
- Vein Removal
- Warts
- Wrinkle Reduction
- User Manuals
- Wavelength
- 10,600 nm (CO2)
- 1060 nm (Diode)
- 1064 nm (Diode)
- 1064 nm (Nd Yag)
- 1064 nm & 755 nm
- 1064 nm, 755 nm & IPL
- 1100 – 1800 nm (Infrared)
- 1319 nm (Infrared Nd Yag)
- 1320 nm (Infrared Nd Yag)
- 1400 nm (Erbium Glass)
- 1440 nm Nd Yag
- 1450 nm (Diode)
- 1470 nm
- 1500 nm (Erbium Glass)
- 1540 nm Erbium Glass
- 1550 nm Erbium Glass
- 1565nm
- 1750nm (Infrared)
- 1927 nm (Thulium)
- 2790 nm (Erbium YSGG)
- 2940 nm (Erbium Yag)
- 308 nm (Xenon Chloride)
- 532 nm (KTP / Q-Switched)
- 585 nm (Pulsed Dye)
- 595 nm (Pulsed Dye)
- 635 nm (Diode)
- 650-660 nm (Diode)
- 670 nm
- 680 nm (Red Diode)
- 694 nm (Ruby)
- 700 nm
- 755 nm (Alexandrite)
- 800 nm (Diode)
- 805 nm (Diode)
- 808 nm
- 810 nm (Diode)
- 850nm (Infrared)
- 900 nm (Diode)
- 980nm (Infrared Diode)
- Acoustic Wave Therapy AWT
- AFT (Advanced Fluorescence Technology)
- AMP – Active Magnetic Pulse
- CO2
- Direct Bio-Electrical Muscle Stimulation
- ElectoFusion
- EMS (Electrical Muscle Stimulation)
- HIFEM (High Intensity Focused
- IPL (Broad Spectrum)
- LED (Light Emitted Diodes)
- LIESWT – Low- Intensity Extracorporeal Shock Wave Therapy
- Multi-Wavelength
- Nitrogen Plasma
- Radio Frequency
- Radio Frequency – Bipolar
- Radio Frequency – Monopolar
- Radio Frequency – Unipolar
- Shock Wave Therapy
- TMA – Thermo-Mechanical Action
- Tripollar RF
- Ultrasound
- Ultrasound & Radio Frequency
Home / Products / Procedure / Hair Restoration / 2023 Venus Concepts ARTAS iX Hair Restoration System with Patient Chair – INCLUDES CONSUMABLE PURCHASING SUPPORT – Free Shipping & Warranty (kmdem)
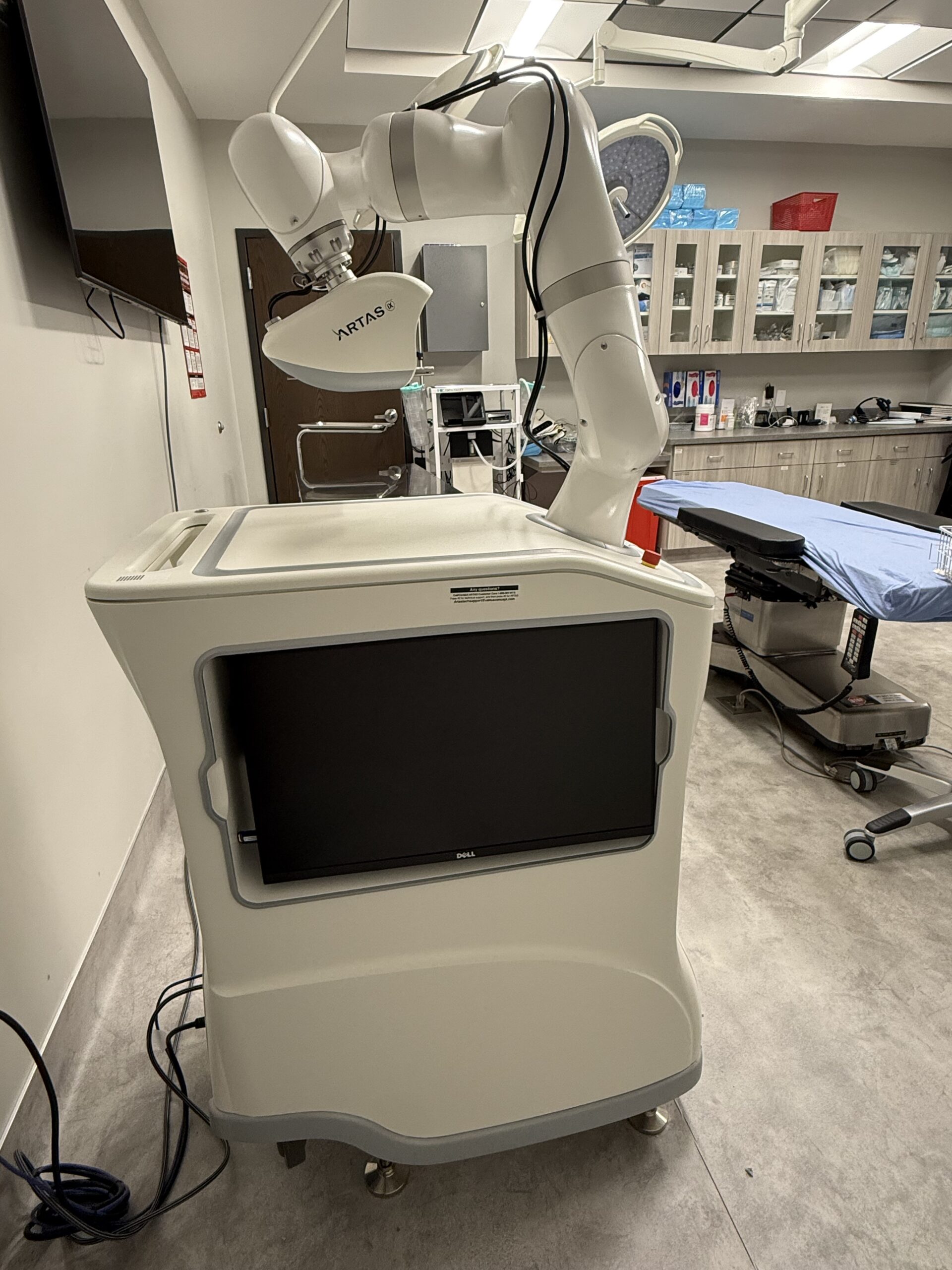
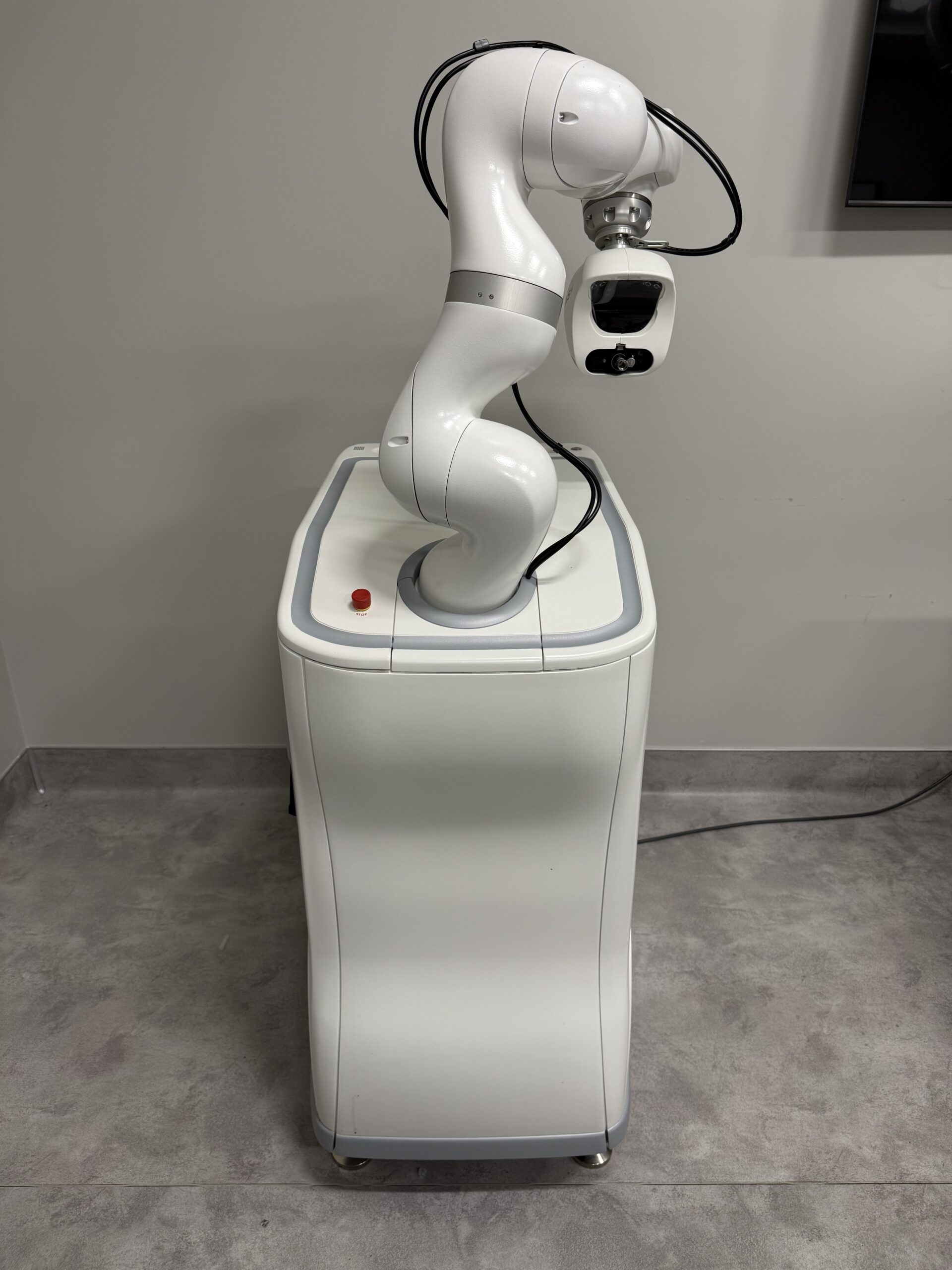
2023 Venus Concepts ARTAS iX Hair Restoration System with Patient Chair – INCLUDES CONSUMABLE PURCHASING SUPPORT – Free Shipping & Warranty (kmdem)
2023 Venus Concepts ARTAS iX Hair Restoration System with Patient Chair;
SELLER HAS AGREED TO REPRESENT THIS DEVICE FOR THE PURCHASE OF CONSUMABLES; NO RECERTIFICATION FEE WILL BE REQUIRED FOR THE PURCHASE OF CONSUMABLES FROM THE MANUFACTURER;
Manufactured 03/23/2023; Excellent Cosmetic and Operating Condition – ONLY USED FOR 13 TREATMENTS; Single Owner; Reason for Selling: No Longer Offering Hair Transplant Services;
Includes: Operator Chair, Key, Operator Manual, 90 Day Warranty, and Free Shipping in the Continental USA & Canada.(km/dem70)
Shipping to Canada $250 Extra; This System Can be Shipped to Anywhere in the World with an Additional Cost.
Standard Free Shipping Includes Freight Curb-Side Delivery. White Glove Delivery Can be Requested at an Additional Cost of $975.
This Equipment is Fully Functional, With No Error Messages, Ready for Use, and Ready to Ship. Actual Photos of the Device are Displayed.
Warranty Includes Cost of Parts, Labor, & Shipping or Travel.
Financing Available. Training is Available at an Additional Cost.
See a Video of this Device Below:
$286,000.00 Original price was: $286,000.00.$85,975.00Current price is: $85,975.00.
Availability: 1 in stock
Artas iX RECERTIFICATION AND CONSUMABLES
Consumables Required:
Harvest Kit: $1,495
Implantation/SM Kit: $395
Needle & Ultra Punch Spares Kit: $495
Bundle of Harvest Kit & Implantation/SM Kit: $1,500
Artas iX PRODUCT SPECIFICATIONS
Harvesting Mechanism: Uses a sharp-blunt dual-punch system with a 7-axis KUKA LBR Med robotic arm for 0.1mm repeatability.
Vision System: Multi-camera stereoscopic vision with 44-micron resolution, capable of analyzing follicles 60 times per second.
Procedural Speed: Capable of harvesting and simultaneously creating sites at a rate of approximately 500 to 700 grafts per hour.
Implantation: Physician-assisted robotic implantation using 25-graft cartridges.
Physical & Technical Data
Dimensions: Length: 34″ | Width: 23″ | Height: 59″.
Weight: 400 lbs (181.4 kg).
Electrical: 100-240 VAC, 50/60 Hz, 15A.
Connectivity: Ethernet enabled for remote diagnostics.
Patient Positioning: Integrated chair-to-table system allows for a “one-room” procedure where the patient does not need to move between harvesting and implantation.
Key Technology in the iX Model
AI Algorithms: Continuously monitors follicle position, angle, and orientation, automatically adjusting for patient movement during the procedure.
ARTAS Hair Studio: A 3D pre-operative planning tool that allows surgeons to design a customized hairline and simulate results for the patient.
Ultra Punch: A proprietary multi-faceted tip designed to minimize follicle trauma during the harvesting phase.
Artas iX MANUFACTURER NOTES
The ARTAS iX is an FDA-cleared, AI-driven robotic platform designed for hair restoration. Manufactured by Venus Concept, it is the only system to automate follicle harvesting, site creation, and implantation in one unit.
Indications for Use
- Harvesting Hair Follicles: Intended for harvesting follicles from the scalp of men diagnosed with androgenic alopecia (male pattern hair loss).
- Patient Requirements: Specifically indicated for patients with black or brown straight hair.
- Procedural Assistance: Intended to assist physicians in identifying and extracting follicular units, creating recipient sites, and implanting the harvested follicles.
Core Capabilities
- The ARTAS iX uses artificial intelligence and high-definition imaging to perform the three major stages of a hair transplant:
- Intelligent Harvesting: A multi-camera stereoscopic vision system with 44-micron resolution scans the scalp 60 times per second to identify and select the most viable hair follicles for extraction.
- Site Creation: It digitally maps the scalp and uses proprietary algorithms to create recipient sites that avoid damaging existing healthy hair while ensuring a natural growth pattern.
- Robotic Implantation: The system can simultaneously create sites and implant follicles, maintaining consistent depth and angle for optimal results.
Patient Benefits
- No Linear Scar: Because it extracts individual units, it eliminates the long linear scar associated with traditional strip (FUT) surgery.
- Precision: AI removes human fatigue and error, ensuring the 1,000th graft is as high-quality as the first.
- Faster Recovery: Most patients can resume normal activities within a few days.
Manufacturer Technical Notes
- AI & Machine Vision: The system uses proprietary AI and a high-definition stereoscopic vision system with 44-micron resolution to analyze follicles 60 times per second.
- Robotic Arm: A 7-axis KUKA LBR Med robotic arm provides 0.1mm repeatability for high-dexterity movements.
- Hardware Precision: It features a 7-axis KUKA LBR Med robotic arm with 0.1mm repeatability for high dexterity during surgery.
- Ultra Punch Technology: Utilizes a multi-faceted tip with a variable coring speed algorithm to minimize trauma to the follicular structure.
- Procedural Speed: The 2023 version (iXi generation) provides up to 25% faster procedures compared to prior models, reaching implantation speeds of up to 1,000 grafts per hour.
- Tensioner System: Features an upgraded “XTensioner” harvest kit designed to simplify follicle harvesting and reduce the need for cleaning reusable parts between sessions.
- Compliance: The device is classified as a Class II medical device, evaluated for biocompatibility under ISO 10993-1, and complies with EN 60601-1 safety standards
* Shipping is included in the Continental USA only. Any shipment outside the lower 48 United States will be shipped at an extra cost. If you live outside this area, please contact us for a free shipping estimate.
Related Products
Related products
-
2021 Capillus PRO 272 – Diode Light Laser Cap for Hair Regrowth / Hair Restoration – Perfect Condition – Free Shipping & Warranty (km/mca)
$2,900.00Original price was: $2,900.00.$1,050.00Current price is: $1,050.00. Add to cart -
2022 Alma TED TransEpidermal Delivery – Non-Invasive Hair Restoration – Scalp Health – DOES NOT INCLUDE CONSUMABLE SUPPORT – Free Shipping & Warranty (jp/jg74-02)s
$180,000.00Original price was: $180,000.00.$15,975.00Current price is: $15,975.00. Read more -
2014 Medicamat Neograft S.A.F.E.R. Automated Hair Restoration System (km/ss)x
$98,000.00Original price was: $98,000.00.$15,975.00Current price is: $15,975.00. Read more